
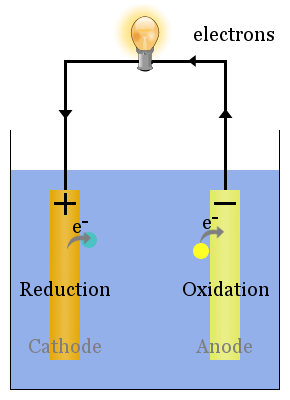
Oxide ions react with oxidized carbon at the anode, producing CO 2(g). The half-reactions that occur at the cathode and the anode are as follows:Įquation 19.109 2Al 2O 3(l) + 3C(s) → 4Al(l) + 3CO 2(g) The signs of the cathode and the anode have switched to reflect the flow of electrons in the circuit. Thus the copper electrode is now the anode (Cu is oxidized), and the cadmium electrode is now the cathode (Cd 2+ is reduced) (part (b) in Figure 19.21 "An Applied Voltage Can Reverse the Flow of Electrons in a Galvanic Cd/Cu Cell"). The applied voltage forces electrons through the circuit in the reverse direction, converting a galvanic cell to an electrolytic cell. We can force the reaction to proceed in the reverse direction by applying an electrical potential greater than 0.74 V from an external power supply. The reverse reaction, the reduction of Cd 2+ by Cu, is thermodynamically nonspontaneous and will occur only with an input of 140 kJ. The anode in an electrolytic cell is positive because electrons are flowing from it, whereas the cathode is negative because electrons are flowing into it. (b) Applying an external potential greater than 0.74 V in the reverse direction forces electrons to flow from the Cu electrode and into the Cd electrode. The potential of the galvanic cell is 0.74 V. (a) When compartments that contain a Cd electrode immersed in 1 M Cd 2+(aq) and a Cu electrode immersed in 1 M Cu 2+(aq) are connected to create a galvanic cell, Cd(s) is spontaneously oxidized to Cd 2+(aq) at the anode, and Cu 2+(aq) is spontaneously reduced to Cu(s) at the cathode.
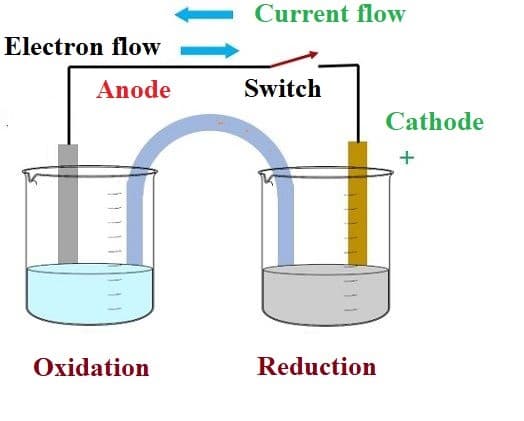
Figure 19.21 An Applied Voltage Can Reverse the Flow of Electrons in a Galvanic Cd/Cu Cell


 0 kommentar(er)
0 kommentar(er)
